A pipeline of next-generation brain-targeting therapies


Our pipeline

Abbreviations: FIH, first in human; GLP, good laboratory practice; IND, investigational new drug; LRRK2, leucine-rich repeat kinase 2.
About Parkinson’s Disease and LRRK2
Parkinson’s disease (PD) is the second most common neurodegenerative disorder among the elderly. While most PD cases occur sporadically, observation of inherited mutations revealed potential molecular pathways underlying neuronal degeneration, offering insights for developing treatments that could change the course of the disease. For example, genome-wide association studies have identified a group of associated risk loci, including the gene encoding leucine-rich repeat kinase 2 (LRRK2). Mutations in the LRRK2 gene have been recognized as genetic risk factors for both familial and sporadic forms of PD, and aberrant kinase activity of LRRK2 mutants has been associated with pathological features of PD. Clinical studies with LRRK2 inhibitors from unique chemical scaffolds have yielded similar safety findings, including lung abnormalities, which suggest on-target effects in the periphery. As a result, there is growing interest in identifying brain-selective LRRK2 inhibitors.
About Tuberous Sclerosis Complex
Tuberous sclerosis complex (TSC) is a rare autosomal dominant genetic disease caused by mutations in the TSC1 or TSC2 genes and resulting in the hyperactivation of the mammalian target of rapamycin (mTOR) pathway. TSC affects 1 in 6,000 live births; prevalence in the United States is approximately 54,000. Epilepsy is one of the most common neurologic symptoms in patients with TSC, with a reported prevalence of approximately 85%, and is a significant cause of morbidity and mortality. Several therapeutic options are available for the treatment of TSC epilepsy, including antiseizure medicines, but a majority of patients develop treatment-refractory epilepsy. Everolimus, a rapamycin analog (rapalog) mTOR inhibitor, is the only FDA-approved mechanistic treatment to reduce intractable seizures. However, 95% of TSC patients who take everolimus report adverse events (AEs), including grade 3 and 4 AEs in 36% of treated patients. This safety profile can lead to dose reductions and discontinuation and even prevent patients from initiating therapy.
How the BrainOnly™ platform works with everolimus, which naturally requires FKBP12 binding for mTOR inhibition
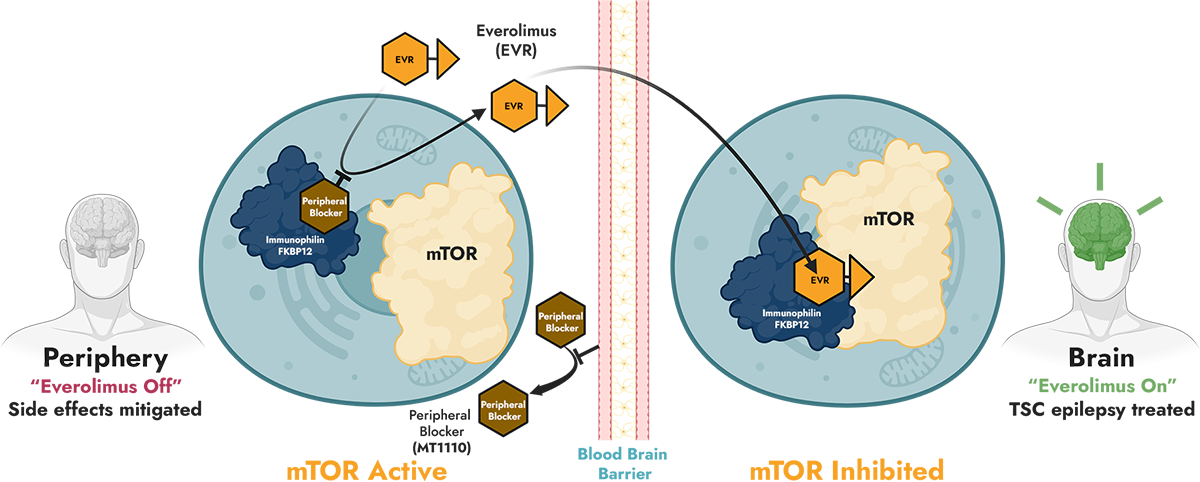
Abbreviation: EVR, everolimus (rapamycin analog); FKBP12, FK506-binding protein 12; mTOR, mammalian target of rapamycin; TSC, tuberous sclerosis complex.


